EUDAMED Registration
In this post, we will go through the information required for EUDAMED Registration and the rationale behind this system.
The European Database on Medical Devices (EUDAMED) is an online platform aimed at placing all actors within the Medical Device industry, within EU onto the same platform.
Actor, in this context, means any organization that needs to register onto the EUDAMED system, namely:
- Manufacturer – a natural, or legal person who manufactures or fully refurbished a device or has a device designed, manufactured, or fully refurbished, and market that device under its name or trademark (Article 2 – section 30).
- Authorized Representative – means any natural or legal person established within the Union who has received and accepted a written mandate from a manufacturer, located outside the Union, to act on the manufacturer’s behalf in relation to specified tasks with regard to the latter’s obligations under this Regulation (Article 2 – Section 32)
- Importer – means any natural or a legal person established within the Union that places ad device from a third country on the Union Market (Article 2 – section 33)
- Systems and Procedure Pack producers – combine devices bearing a CE marking with the following other devices or products, in a manner that is compatible with the intended purpose of the devices or other products and within the limits of use specified by their manufacturers, in order to place them on the market as a system or procedure pack (Article 22 (1)).
- Distributor – any natural or legal person in the supply chain, other than the manufacturer or the importer, that makes a device available on the market, up until the point of putting it into service (Article 2 – Section 34).
Modules within the EUDAMED System
After completing the EUDAMED Registration, you will have the following modules:
EUDAMED Registration for EOs (Article 30)
All the above are considered as EO (Economic Operators). And from the above EOs, only distributors don’t need to register with EUDAMED. Having said that, they will need to Register as EOs with the Medicines Authority.
Each EO will be allocated an SRN (Single Registration number) so that they can all be identified correctly. Should a company do more than 1 role (for example manufacturer and importer), they would need to register for 2 separate SRNs. An example of an SRN is as follows: MT-MF-1234567890:
- MT – is the country code
- MF – the type of EO
- 1234567890 – a unique number generated automatically by the EUDAMED system.
Moreover, distributors still have their own requirements within the Medical Device Regulations, and still have to follow GDP as defined in Malta.
It is the responsibility of the importer to ensure that the manufacturer has updated the EUDAMED system (within 2 weeks of placing the device on the market). Moreover, should there be any missing or incorrect information, they should notify the manufacturer accordingly.
UDI and devices registration (Article 28 & 29 (4))
UDI refers to a Unique Device Identification and is comprised on the following:
- UDI-DI – (device identifier) is specific to the manufacturer and a device. This is static and doesn’t change as it represents a specific device from your product range.
- UDI-PI – (production identifier) refers to the production dates and packaged devices. This is dynamic, and changes with every batch of the production run.
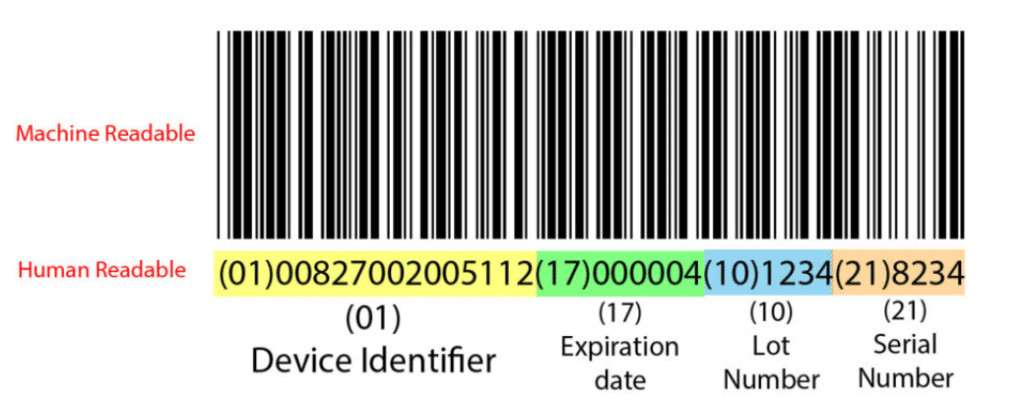
Naturally, it is the responsibility of the manufacturer to assign a UDI to a specific product. It is important not to confuse the UDI-DI with the Basic UDI-DI. The latter is an identification number for a group of products and has no real use for traceability. All products within your (the manufacturer’s) range that have the same categories would have the same Basic UDI-DI:
- intended purpose
- risk class
- essential design
- manufacturing characteristics
The basic UDI doesn’t show on the product and/or packaging, and will only appear on:
- certificates by notified bodies
- DoC (declaration of conformity)
- Technical Documentation
- Summary of safety and clinical performance
The European Commission launched a new helpdesk on 17 May 2021 to assist economic operators in implementing the new obligations and requirements introduced by the new UDI system.
The helpdesk can be reached out following this link.
The UDI helpdesk provides support on UDI assignment, labeling, and registration of devices. It also provides support on the use of the European Medical Devices Nomenclature (EMDN), which the European Commission has made available to manufacturers and other natural or legal persons required by the MDR and IVDR to use it.
Notified bodies and certificates (Article57)
A thorough explanation of the role of Notified bodies is beyond the scope of this article. However, it is important to note that notified bodies are a critical aspect of the MDR (and IVDR) as they are able to grant the CE mark to a device, before placing it on the market.
It is extremely interesting to note that in Malta, we have 2 notified bodies out of the total of 16 that are available within the EU.
Clinical investigations (Article 73)
A patient-centric approach to healthcare is vital. Clinical trials are required to determine the comparative efficacy of two or more therapies. The EUDAMED system is to serve as the main electronic system for clinical investigations.
Market surveillance (Article 100)
Market surveillance in the context of medical devices, and more specifically EUDAMED, is the enforcement of the harmonized legislation on medical devices.
Vigilance and post-market survelliance (Article 92)
The overall concept behind the NDR is that of ensuring safe products on the EU market. Even though all precautions and measures are taken, certain mistakes might still happen. Through the EUDAMED system, a centralized location for the handling of corrective actions will be in place.
EUDAMED Registration
To complete the registration for EUDAMED Registration, the following information is required:
- The legal name of the company
- The VAT Number
- EORI (Economic Operators Registration Identification) – Malta Customs Authority assigns a unique identifying number, known as EORI, to each and every trader who interacts with Customs. In Malta, this is the same as the VAT Number.
- Signed declaration – downloaded here